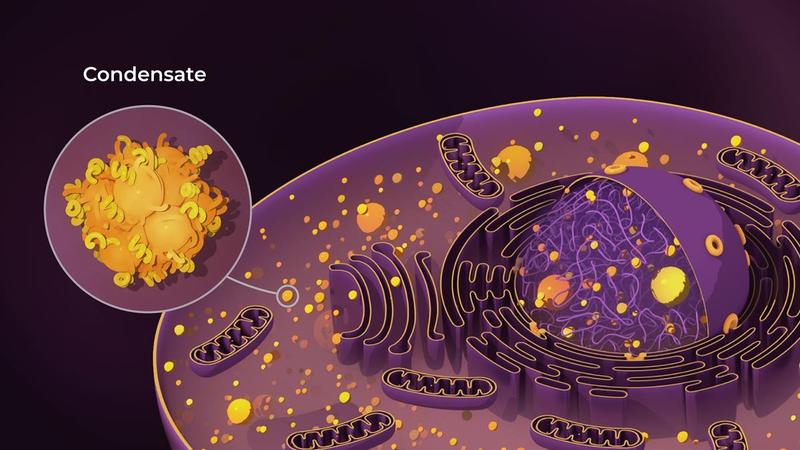
From descriptive cell models to predictive digital representations
The concept of digital cell twins is emerging as a cornerstone of next-generation cell biology. Unlike traditional computational models that retrospectively describe experimental observations, digital cell twins aim to create predictive virtual counterparts of living cells, capable of simulating how cells behave under defined and evolving conditions.
At their core, digital cell twins integrate biological data with computational modeling to represent not only cellular identity, but also cellular dynamics. These models are designed to anticipate phenotypic changes in response to biochemical signals, mechanical constraints, and environmental perturbations. This shift reflects a broader transformation in life sciences, moving from static representations toward data-driven, forward-looking biological systems.
Why 3D cell culture demands predictive modeling
Three-dimensional cell culture systems have become essential for studying complex biological phenomena that cannot be captured in two-dimensional environments. By enabling spatial organization, cell–cell interactions, and tissue-like architectures, 3D systems offer improved physiological relevance. However, they also introduce layers of complexity that challenge experimental control and interpretation.
Cells in 3D culture are exposed to heterogeneous microenvironments. Nutrient and oxygen gradients develop over time. Mechanical forces vary locally. Cell populations evolve dynamically as aggregates grow, reorganize, or differentiate. These non-linear behaviors make it difficult to isolate causal relationships using empirical experimentation alone.
Digital cell twins provide a framework to decode this complexity. By simulating how cells respond to spatial, temporal, and mechanical variables, virtual models help disentangle the interplay between system parameters and biological outcomes. In this context, digital twins act as interpretive lenses, transforming complex 3D cultures into intelligible systems.
Integrating multi-omics and physical parameters
A defining feature of digital cell twins is their ability to integrate multiple layers of biological information. Genomic data define the genetic blueprint of the cell. Transcriptomic profiles capture regulatory states and gene expression dynamics. Proteomic and metabolomic layers reflect functional activity and metabolic adaptation.
Crucially, these molecular dimensions are coupled with physical and environmental variables. Nutrient availability, oxygen diffusion, extracellular matrix properties, spatial confinement, and mechanical forces are incorporated as dynamic inputs rather than static background conditions. This integration acknowledges that cellular behavior emerges from the continuous interaction between molecular programs and physical context.
Rather than producing a single static model, digital cell twins evolve through iterative refinement. Experimental measurements inform computational updates, which in turn generate new hypotheses to be tested experimentally. This feedback loop progressively increases model fidelity, enabling virtual cells to approximate real biological behavior more closely over time.
Relevance for sensitive and complex cell systems
Digital cell twins are particularly valuable for systems where small variations in culture conditions produce disproportionate biological effects. Fragile, shear-sensitive, or highly plastic cells often exhibit strong sensitivity to mechanical stress, nutrient fluctuations, or spatial constraints. In such cases, exhaustive experimental exploration of parameter space is impractical.
Virtual modeling enables researchers to explore “what-if” scenarios computationally, identifying critical thresholds and interactions before committing to physical experiments. This capability reduces empirical trial-and-error while increasing insight into the mechanisms driving observed phenotypes.
Guiding experimental design in advanced 3D cultures
Importantly, digital cell twins are not positioned as replacements for experimental platforms. Their primary value lies in guiding experimental design. By highlighting influential parameters and predicting system-level behavior, digital twins help prioritize experiments with the highest expected information gain.
In advanced 3D culture systems, predictive modeling can inform decisions related to cell density, aggregation kinetics, culture duration, oxygenation strategies, and acceptable mechanical stress ranges. This guidance supports more rational design of experiments and contributes to improved reproducibility across laboratories and platforms.
When combined with controlled 3D culture technologies, digital cell twins enable tighter alignment between biological intent and physical implementation. Computational insight and experimental control reinforce one another, reducing uncertainty at each stage of development.
Implications for scalable and standardized cell biomanufacturing
As cell-based systems transition from research tools to translational and industrial applications, predictability and scalability become critical requirements. Variability that is tolerable in exploratory research becomes a liability in manufacturing contexts.
Digital cell twins offer a pathway toward more standardized workflows by enabling anticipation of variability rather than reactive correction. By modeling how cells respond to changes in scale, environment, and process parameters, virtual twins support the design of robust operating windows that preserve biological function.
This predictive capability aligns with the broader evolution of cell biomanufacturing toward model-informed process development, where computational tools complement experimental validation to accelerate development while maintaining quality.
Toward intelligible and controllable 3D cell systems
The rise of digital cell twins signals a conceptual shift in how complex cellular systems are approached. Instead of accepting biological variability as an unavoidable limitation, predictive modeling reframes variability as a phenomenon that can be understood, anticipated, and partially controlled.
In this vision, 3D cell culture becomes not only more physiological, but also more intelligible. Digital twins provide a bridge between biological complexity and engineering logic, enabling in vitro systems that are reproducible, scalable, and aligned with future demands in research and biomanufacturing.
As virtual cell models continue to mature, they are poised to become integral components of next-generation experimental platforms, supporting a deeper and more predictive understanding of cellular behavior in complex environments.